Radiomics
Dr Henry Knipe◉◈ and Dr
Muhammad Idris◉ et al. From Radiopaedia
Radiomics (as applied to radiology)
is a field of medical study that aims to extract a large number of quantitative
features from medical images using data characterization algorithms. The data
is assessed for improved decision support. It has the potential to uncover
disease characteristics that are difficult to identify by human vision alone.
Process
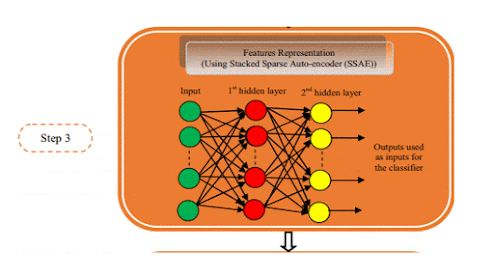
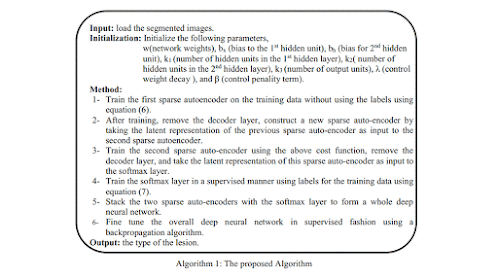
The process of creating a database of correlative quantitative
features, which can be used to analyze subsequent (unknown) cases includes the
following steps 3.
Initial
image processing
Using a variety of reconstruction algorithms such as contrast,
edge enhancement, etc. This influences the quality and usability of the images,
which in turn determines how easily and accurately an abnormal characteristic
could be detected and characterized.
Image
segmentation
Identify/create areas (2D images) or volumes of interest (3D
images). Can be done either manually, semi-automated, or fully automated
using artificial intelligence.
For large data sets, an automated process is needed because
manual techniques are usually very time-consuming and tend to be less accurate,
less reproducible and less consistent compared with automated artificial intelligence techniques.
Features
extraction and qualification
Features include volume, shape, surface, density, and
intensity, texture,
location, and relations with the surrounding tissues.
Semantic features are those that are commonly used in the
radiology lexicon to describe regions of interest.
Agnostic features are those that attempt to capture lesion
heterogeneity through quantitative mathematical descriptors.
Examples
of semantic features
·
shape
·
location
·
vascularity
·
spiculation
·
necrosis
·
attachments
Equivalent
examples of agnostic features
·
histogram (skewness, kurtosis)
·
Haralick textures
·
Laws textures
·
wavelets
·
Laplacian transforms
·
Minkowski functions
·
fractal dimensions
Uses
Radiomics can be applied to most imaging modalities including
radiographs, ultrasound, CT, MRI and PET studies. It can be used to increase
the precision in the diagnosis, assessment of prognosis, and prediction of
therapy response, particularly in combination with clinical, biochemical, and
genetic data. The technique has been used in oncological studies, but
potentially can be applied to any disease.
A typical example of radiomics is using texture analysis to
correlate molecular and histological features of diffuse high-grade
gliomas 2.
The determination of most discriminatory radiomics feature
extraction methods varies with the modality of imaging and the pathology
studied and is therefore currently (c.2019) the focus of research in the field
of radiomics.
Current challenges include the development of a common
nomenclature, image data sharing, large computing power and storage
requirements, and validating models across different imaging platforms and
patient populations.
References
Promoted
articles
1.
MAPS:
A Quantitative Radiomics Approach for Prostate Cancer Detection
EMBS Trans Biomed
Eng, 2015
2.
Robust
Collaborative Clustering of Subjects and Radiomic Features for Cancer Prognosis
EMBS Trans Biomed
Eng, 2020
EMBS J Biomed Health
Inform, 2018
1.
Multi-Objective-Based
Radiomic Feature Selection for Lesion Malignancy Classification
EMBS J Biomed Health
Inform, 2019
2.
Benefits
and limitations of real-world evidence: lessons from EGFR mutation-positive
NSCLC
Future
Oncology, 2020
3.
A
Radiomics Approach With CNN for Shear-Wave Elastography Breast Tumor
Classification
EMBS Trans Biomed
Eng, 2018