SIÊU ÂM TOÀN THÂN TRONG CẤP CỨU.
Tổng số lượt xem trang
Thứ Bảy, 7 tháng 9, 2019
Chủ Nhật, 1 tháng 9, 2019
DÀY QUANH KHOẢNG CỬA= CHỈ DẤU BỆNH LÝ VIÊM TRONG Ổ BỤNG TRẺ EM
Is the presence of echo-rich periportal cuffing in the liver indicator
for abdominal inflammation in pediatric patients?
Nurdan Fidan1, Esra Ummuhan Mermi Yetis2, Muammer Murat1, Cuneyt Yucesoy3, Ebru Turgal4, Mehmet Metin5
Nurdan Fidan1, Esra Ummuhan Mermi Yetis2, Muammer Murat1, Cuneyt Yucesoy3, Ebru Turgal4, Mehmet Metin5
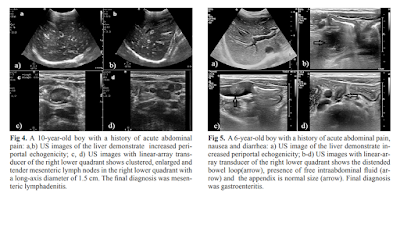
ErPC [echo rich periportal cuffing] appears especially in diseases
associated with abdominal inflammation such as gastroenteritis, acute
appendicitis, perforated appendicitis and mesenteric lymphadenitis. When
evaluated with adequate clinical information, the presence of ErPC is a
finding of high sensitivity and specificity in pediatric patients. This could
have a significant contribution to correct diagnoses by directing the
radiologists or clinicians to further examinations or follow-ups.
DÀY KHOẢNG CỬA: DẤU HIỆU SIÊU ÂM CỦA VIÊM TRONG Ổ BỤNG
MỤC TIÊU HỌC TẬP:
1/ Mô tả được hình ảnh siêu âm dày khoảng cửa và dấu trời sao khi
khám siêu âm gan
2/
Trình bày được các nguyên nhân và tình huống lâm sàng của dày khoảng cửa và dấu
trời sao trong siêu âm gan
3/ Phân tích được một số tình huống lâm sàng có dày
khoảng cửa và dấu trời sao
NỘI DUNG
1. DẪN NHẬP:
Đau bụng
là lý do để khám siêu âm bụng trong thực hành lâm sàng ngoài các cách khám lâm
sàng truyền thống. Tuy không là phương tiện chẩn đoán chính thống, siêu âm bụng
thường quy/tại giường/tiếp cận giúp định hướng chẩn đoán và cung cấp hình ảnh
bình thường và bệnh lý trong khoang bụng.
Gần
đây y văn siêu âm có những bài báo về đau bụng ở trẻ em và người lớn, trong đó
ghi nhận vài dấu hiệu siêu âm TRONG GAN như phản ứng quanh cửa [portal
reaction], dấu trời sao [starry sign appearance], dày quanh cửa [periportal
echo rich] trong một số bệnh lý như viêm gan, viêm dạ dày ruột, viêm ruột dư cấp…
Trong
bài này chúng ta sẽ mô tả và tìm hiểu các dấu hiệu siêu âm trên.
2. CÁC DẤU HIỆU SIÊU ÂM TRONG GAN CỦA ĐAU BỤNG:
A. PHẢN ỨNG QUANH CỬA [PORTAL REACTION]:
Là các đường echo rich cạnh khoảng cửa gan trên nền
nhu mô gan echo poor thường gặp trong viêm gan cấp (từ 10% đến 13.9% , n=1.367 ca)
được cho là các tế bào gan viêm phù nề làm tăng sáng quanh cửa ở trung tâm gan.
Dấu hiệu này có thể gặp trong các ổ bụng có hội chứng viêm các cơ quan khác như
dạ dày,ruột.
|
|
B. DDẤU TRỜI SAO [STARRY SKY APPEARANCE]:
Là các đường echo rich rải rác trong vùng ngoại biên
gan. Dấu hiệu này có thể gặp trong các ổ bụng có hội chứng
viêm các cơ quan như gan, dạ dày,ruột. Theo H Tchelepi -
2002, dấu hiệu này không có giá trị trong viêm gan
C. DÀY QUANH CỬA [PERIPORTAL ECHO RICH]:
Dấu hiệu này mới được công bố trong một bài báo siêu
âm ở trẻ em (2019). Tác giả không ghi nhận có trong viêm gan, chỉ có ở một số
trường hợp viêm trong ổ bụng ở trẻ em cả 2 dấu trời sao và dày khoảng cửa.
|
|
3. BÀN LUẬN:
Cả 3 dấu hiệu này đều ở khoảng quanh cửa trung tâm và
ngoại biên của gan. Đây là mô tả của siêu âm dựa vào phù hợp của chẩn đoán lâm
sàng sau cùng, chưa được chứng minh bằng các phương tiện chẩn đoán hình ảnh
khác.
Khi tìm thấy 3 dấu hiệu trên người làm siêu âm nên tìm
thêm các triệu chứng siêu âm bất thường ở gan, dạ dày, ruột, hạch trong ổ bụng
để giúp hướng chẩn đoán cho một trường hợp đau bụng trong thực hành lâm sàng.
TÀI LIỆU THAM KHẢO:
SIÊU ÂM CHẨN ĐOÁN TRONG VIÊM GAN, 1997, Nguyễn Thiện Hùng – Phan
Thanh Hải – Phạm Thi Thu Thủy Trung tâm Chẩn đoán Y khoa (MEDIC) Thành Phố Hồ
Chí Minh
SONOGRAPHY of DIFFUSE LIVER DISEASES, by H Tchelepi -
2002, www.jultrasoundmed.org/content/21/9/1023.full
STARRY SKY APPEARANCE
https://radiopaedia.org/articles/starry-sky-appearance-ultrasound-1
IS THE PRESENCE OF
ECHO-RICH PERIPORTAL CUFFING IN THE LIVER INDICATOR FOR ABDOMINAL INFLAMMATION
IN PEDIATRIC PATIENTS htps://www.medultrason.ro/medultrason/index.php/medultrason/article/view/1940
Thứ Sáu, 23 tháng 8, 2019
FDA adds tranducer check to ultrasound guidance.
By Kate Madden Yee, AuntMinnie.com staff writer
August 23, 2019 -- New ultrasound transducers should include an integrated quality control check that runs every time the probe is turned on, according to final guidance issued by the U.S. Food and Drug Administration (FDA) on August 22
The FDA plans to incorporate the new transducer check recommendation into the agency's 510(k) compliance policy that covers marketing clearance of diagnostic ultrasound systems and transducers. The new policy -- as well as clarifications to guidelines on when the FDA will require modified probes to receive a new 510(k) application -- was explained in a webinar by Shahram Vaezy, PhD, of the FDA's Center for Devices and Radiological Health.
"This guidance supersedes the FDA's 2008 guidance ... and describes the types of modifications to a diagnostic ultrasound device for which the FDA does not intend to enforce the requirement for a premarket notification," Vaezy said. "[It also] includes a new transducer element check that applies to all ultrasound devices."
Transducer element check
The FDA is recommending that manufacturers integrate into transducers for which they are filing new 510(k) applications a way to test probe performance each time it is activated.
"Each device should include some level of testing," the agency wrote. "This integrated test feature would also generate a report on the performance of the probe under test for documentation ... [and] should also be available to the operators to initiate anytime when a particular probe is suspected of failure."
Developing transducer check features will take time, Vaezy acknowledged.
"The FDA is open to discussions with manufacturers about how to implement transducer checks into devices," he said.
Compliance policy
The FDA clarified that it will not require a new 510(k) for modified ultrasound and transducer devices that have already received an initial 510(k) clearance when all of the following apply:
- The intended use of the modified device is not changed.
- The device is not a reusable device subject to the requirement for the submission of reprocessing labeling and validation data.
- The modes of operation for the modified device are well-established.
- The modifications do not lead to acoustic outputs that exceed the recommended maximum acoustic output levels.
- The modifications do not result in a range of ultrasound interrogation parameters outside a well-known range.
- The modifications do not use novel mechanical or thermal effects for imaging or measurements.
- The measurements and analyses are clearly described, and the user can adjust the associated control parameters.
- Transducer element check is performed.
- Transducer surface temperature falls within a well-defined range.
- Appropriate transducer covers are recommended to users.
During the webinar, Vaezy listed some examples of possible compliance policy applications that would require a new 510(k):
- Adding continuous-wave and pulsed-wave Doppler methods to the device
- Adding an algorithm that measures the volume of an organ based on established image segmentation and volume calculation methods
- Adding a new transducer with similar indications for use and similar acoustic output
- Adding a B-mode noise reduction filter for general imaging use to a system
"The revised final guidance enables manufacturers with an established track record of ultrasound device development, via submission of 510(k)s for their original devices, to add new safety features and make certain modifications to address clinical needs without [having to] submit another 510(k)," he said.
Thứ Sáu, 16 tháng 8, 2019
USPSTF Opens Review of carotid stenosis screening.
August 16, 2019 -- The U.S. Preventive Services Task Force (USPSTF) has posted a draft research plan on screening for asymptomatic carotid artery stenosis, an exam that typically involves the use of ultrasound to detect signs of stenosis before a stroke can occur.
The new plan is part of the USPSTF's five-year review of its previous guidance on carotid artery screening, issued in 2014. At that time, the USPSTF gave the procedure a letter grade of D, recommending against its use in asymptomatic individuals.
The low prevalence of carotid artery stenosis would result in many false positives on screening ultrasound, the group concluded in 2014. In addition, a large number of surgical interventions such as carotid endarterectomy could be performed that aren't any better than standard medical therapies such as statins in terms of reducing stroke risk, according to the task force.
The new draft introduces the following questions for review:
- Is there direct evidence that screening asymptomatic adults for carotid artery stenosis with duplex ultrasonography improves health outcomes?
- What are the harms associated with screening for asymptomatic carotid artery stenosis?
- For asymptomatic persons with carotid artery stenosis, does treatment with carotid endarterectomy or carotid angioplasty and stenting provide incremental benefit beyond current standard medical therapy?
- What are the harms associated with carotid endarterectomy or carotid angioplasty and stenting for the treatment of asymptomatic carotid artery stenosis?
The draft research plan is available for public comment from August 15 through September 11, 2019.
ElastoUS methods reduce unnecessary breast biopsies
By Kate Madden Yee, AuntMinnie.com staff writer
August 15, 2019 -- Using a combination of different elastography methods reduces unnecessary biopsies of BI-RADS 4 lesions without increasing the risk of missing cancers, according to a new study published in the September issue of Ultrasound in Medicine and Biology.
The findings suggest a better way to deal with BI-RADS 4 lesions, which can be tricky to assess for their potential to become malignant, according to a team led by Dr. Jing Han of Sun Yat-Sen University Cancer Center in Guangzhou, China.
"BI-RADS category 4 lesions exhibit a broad range of malignant potential (2% to 95%)," the group wrote (Ultrasound Med Biol, September 2019, Vol. 45:9, pp. 2317-2327). "Therefore, it is necessary to develop a noninvasive and reliable method to discern low-risk lesions as a complement to conventional ultrasound to reduce the unnecessary biopsy rate. Elastography has potential to serve as this valuable tool."
A key modality
Ultrasound is an important modality for breast cancer screening and differentiating benign from malignant lesions, but high false-positive rates can lead to unnecessary biopsies -- making the problem of reducing biopsy of benign lesions without missing cancers a key clinical issue, according to Han and colleagues. Ultrasound elastography may be just the ticket, they noted.
"Ultrasound elastography is beneficial for differentiating breast lesions in many studies," the team wrote. "This technique, which is sensitive to tissue stiffness, has been conducted as a complementary modality for improving breast lesion characterization."
There are a number of elastography techniques: strain (SE), shear wave (SWE), virtual touch imaging (VTi), and virtual touch imaging quantification (VTIQ). Each has its benefits and limitations, and studies have shown that using conventional ultrasound with a single elastography technique can reduce false-positive rates but also increase false-negative rates. Han and colleagues sought to investigate what combinations of strain elastography, VTi, and VTIQ might reduce false positives without increasing false negatives in evaluating BI-RADS category 4 lesions.
The study included 267 patients with 278 BI-RADS 4 lesions who were scheduled for ultrasound-guided biopsy between January 2016 and May 2017. Of the 278 lesions, 151 were benign and 127 were malignant. All were evaluated with conventional B-mode ultrasound, strain elastography, SWE, VTi, and VTIQ (SWE was used with VTIQ; the researchers did not break it out as an individual elastography category for assessment).
Han and colleagues evaluated the following factors: diagnostic performance (including area under the receiver operating characteristic curve, or AUC) sensitivity, specificity, accuracy, positive predictive value (PPV) and negative predictive value (NPV).
The group found that VTi alone showed the highest negative predictive value at 91.7%, although combined methods showed higher negative predictive value than single methods, with the highest negative predictive value at 100% when VTi, SE, and VTIQ were combined.
Han's team also found that, compared with conventional ultrasound, positive predictive value increased from 45.7% to 63.1% when combined elastography was added (VTi plus SE plus VTIQ). Of the BI-RADS 4 lesions, 52.5% were downgraded when using a combination of VTi plus SE, and 50.8% were downgraded when using VTi plus strain elastography plus VTIQ -- without missing any cancer.
| Performance of elastography techniques for reducing unnecessary biopsy of BI-RADS 4 breast lesions | |||||||
| Performance measure | SE | VTi | VTIQ | VTIQ plus VTi | VITQ plus SE | VTi plus SE | VTi, SE, & VTIQ |
| Sensitivity | 89.7% | 92.1% | 85% | 96.8% | 98.4% | 99.2% | 100% |
| Specificity | 63.5% | 73.5% | 84.7% | 67.5% | 59.6% | 52.9% | 50.9% |
| Accuracy | 75.5% | 82% | 84.9% | 80% | 77.3% | 74.1% | 73.4% |
| PPV | 67.4% | 74.5% | 82.4% | 71.5% | 67.2% | 63.9% | 63.1% |
| NPV | 88% | 91.7% | 87% | 96.2% | 97.8% | 98.7% | 100% |
| AUC | 0.82 | 0.84 | 0.90 | 0.82 | 0.79 | 0.76 | 0.75 |
As for statistical significance, the group found that the specificity and area under the receiver operating curve of VTIQ were significantly higher than those of SE and VTi, and the sensitivity of combined methods was significantly higher than single methods.
Effective combinations
More research is needed to determine the role of combined elastography techniques in reducing unnecessary biopsies of BI-RADS 4 breast lesions, according to Han and colleagues.
"Our initial clinical result revealed that the combination of different types of elastography could improved the sensitivity and negative predictive value, which might serve as a complementary approach to conventional ultrasound to ... reduce unnecessary biopsy for benign breast lesions," the team concluded. "Further studies with a larger population are required to validate our results."
Đăng ký:
Nhận xét
(
Atom
)