The secrets of the abdomen
Overview
of abdominal point-of-care ultrasound use in the ICU, potential diagnoses and
findings common to the critical care patient population.
The use of point-of-care ultrasound (POCUS) in
critical care as a diagnostic and monitoring tool is rapidly expanding. While
its role in cardiovascular and respiratory assessment is well established
(within critical care), abdominal ultrasonography is less so; perhaps because
of the myriad of potential diagnoses that can be made and the fact that the
abdomen is often less accessible due to gaseous interposition. Regardless of
modality, the key difference between radiology department scans and scans
performed by intensivists is that the latter are more focused and aim to answer
a specific clinical question in the context of a specific clinical situation.
It is without the scope of this article to describe
every single (potential) use of abdominal POCUS; the aim is to provide an
overview of the potential diagnosis and findings common to the critical care
patient population.
Basic B-mode ultrasound
1. Trauma
One of the earliest uses of ultrasound (US) outside of
radiology was in the detection of intra-abdominal free fluid (or blood) in the
context of trauma. The Focused Assessment with Sonography in Trauma (FAST) scan
has been consistently included as part of the Advanced Trauma Life Support
(ATLS) course over the latest editions (Royal College of Surgeons 2017). The
original FAST scan included assessment of the hepato-renal recess (right upper
quadrant a.k.a Morison’s pouch), the spleno-renal recess (left upper quadrant)
and the pelvis for the presence of free fluid/blood (Carroll et al. n.d.).This
has been expanded to include the subcostal views (pericardial fluid/tamponade),
anterior thoracic views (to rule out pneumothorax) and the detection of pleural
fluid in the so-called extended FAST or eFAST (123sonography.com n.d.) (Figure
1).

The
sensitivity and specificity of FAST for the detection of free intraperitoneal
fluid were 64–98% and 86–100%, respectively (Bloom and Gibbons 2018). This
range may be explained by differences in the levels of clinical experience and
in the reference standards.
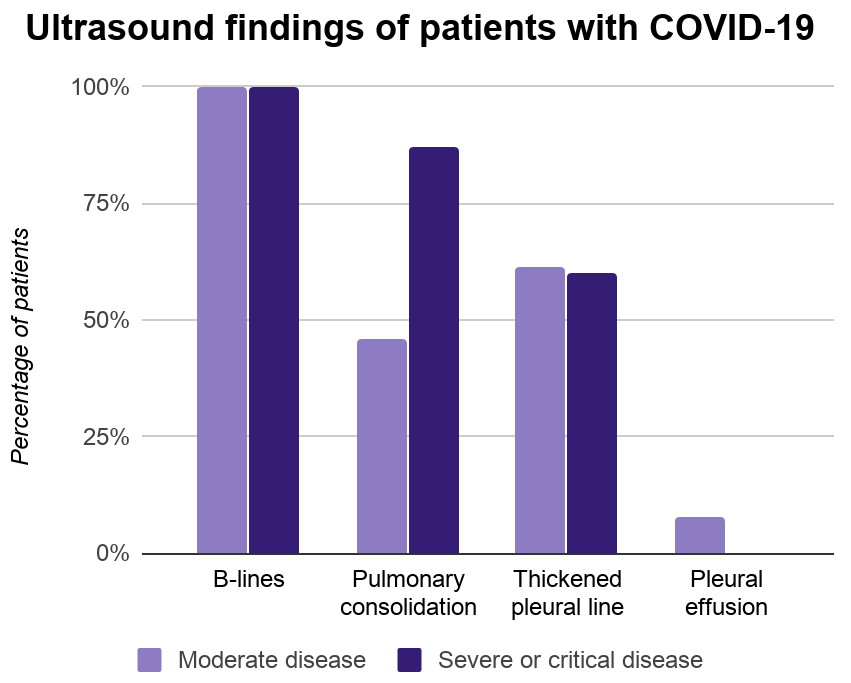
2. Abdominal free fluid
Ultrasound allows for the identification of free fluid,
quantification of the volume and potentially, the underlying aetiology.
The differential diagnosis of the presence of abdominal free
fluid is summarised in Table 1. In the critically ill the main cause for
abdominal fluid is in the setting of sepsis, capillary leak and massive fluid
resuscitation, as seen in severely burned patients. The aetiology of
spontaneous haemoperitoneum can vary, and the causes may be classified as
gynaecologic, hepatic, splenic, vascular, or coagulopathic conditions.
US
is not sensitive at identifying a focus of extravasation from a vessel or organ
(Schmidt et al. 205). Therefore, FAST may be an option for the initial
evaluation of a patient to detect haemoperitoneum in non-trauma patients, but
it does not replace computed tomography (CT) scanning.
3. Assessment of gastric content
Dysfunctional gastric emptying in critically ill patients can
contribute to complications during procedures related to airway management and
can result in unsuccessful enteral feeding as well as an increased risk of
aspiration (Marik 2001). A 6-hour fasting period (2 hours for clear fluid) has
been recommended for patients undergoing elective surgery to reduce the risk of
aspiration during anaesthesia (s.n. 2017). In the ICU, gastric emptying is
frequently altered and influenced by several factors, including age, diagnosis
on admission (Hsu et al. 2011) underlying disease processes (e.g. diabetes,
porphyria, shock)(Nguyen et al. 2007), therapeutic interventions (e.g.
mechanical ventilation), medications (e.g. opioids, sedatives, neuromuscular
blockers, vasopressors) (Nimmo et al. 1975; Steyn et al. 1997), electrolyte and
metabolic disturbances and mechanical ventilation (Mutlu et al. 2001).
Epidural anaesthesia, on the contrary, improves gastric emptying
and peristalsis. The measure of the antral cross-sectional area (CSA) by US is
feasible in most critically ill patients and would allow for direct
visualisation of stomach content. On average, a CSA > 15-25 cm2 corresponds
to a gastric residual volume (GRV) > 300 mL. The same principle has been
studied with regard to assessment of preoperative fasting status amongst
surgical patients (Van der Putte and Perlas 2014; Perlas et al. 2009).
Gastric US can also identify other pathologies such as gastric
tumours (carcinomas and rarely teratomas), hypertrophic pyloric stenosis and
even bezoar related to enteral nutrition.
Normal stomach wall anatomy consists of five layers, referred to
as the gut signature:
- Serosa (hyperechogenic)
- Muscularis propria (hypoechogenic)
- Submucosa (hyperechogenic)
- Muscularis mucosa (hypoechogenic)
- Mucosa (hyperechogenic)
4.
Bowel obstruction
Features of the bowel which can be assessed using US
include:
- Wall thickness
- Diameter and intraluminal contents
- Peristalsis
- Vascularity
The diameter of the bowel and its contents may vary
according to site, fasting/feeding state and bowel function. In adults, the
normal small bowel measures under 30mm in diameter and the normal large bowel under 60 mm in diameter (Reintam Blaser et al. 2012). Dilated loops may show
thickened walls (normally up to 3 mm in the small bowel), or thickened valvulae
conniventes (normally up to 2mm in the large bowel). The exceptions to this are
the duodenal bulb and rectum, which are less than 3 and 4mm in thickness
respectively (Lichtenstein et al. 2014).Ultrasound patterns can aid in the
differentiation of small from large bowel (Table 2).

Assessment of bowel peristalsis is difficult and
subjective, but may provide useful information in several intestinal diseases.
Increased small bowel peristalsis has been described in coeliac disease and
acute mechanical bowel obstruction (increased to-and-fro motion of the bowel
contents) (Hefny et al. 2012). In later phases, one may detect a fluid-filled
lumen, thinning and spasm of the bowel wall, evidence of extraluminal fluid and
decreased or absent peristalsis.
Types of peristalsis:
- Absent peristalsis
- Present ineffective peristalsis
- Present effective peristalsis
- Augmented peristalsis
5. Viscus perforation
Physiologic air can be seen in the lumen of the bowel
as small stars. Larger air bubbles can appear as hyperechoic stripes generating
comet tail artefacts (these are rare in the small bowel but frequent in the
large bowel), much like a linear view of the lung would look. Air artefacts can
emanate from the thoracic cavity and the lung over the liver. Pathological air,
however, may produce an enhanced peritoneal stripe sign (EPSS), reverberation
artefacts and ring-down artefacts (Figures 2a and 2b) (Hoffmann et al. 2012).
6. Renal dysfunction
International guidelines recommend that all patients
who present in acute renal failure undergo an ultrasound examination to
ascertain its cause (Kidney Disease: Improving Global Outcomes 2012).
Hydronephrosis due to obstructive uropathy is a reasonably straightforward
diagnosis to make.
More advanced techniques include the assessment of
blood flow within the renal artery and vein using Doppler analysis.
Analysis of the urinary bladder should be performed as
well. The bladder can be empty, filled or distended (globus). The position of
the bladder catheter balloon can be checked, as well as the presence of
hyperechogenic structures (debris, tumour, blood clot etc).
7. Liver and spleen
Ultrasound of the liver is divided into general US
views including anatomic views of the liver, gallbladder and biliary tree.
Pathology within these organs e.g. acute liver failure, can result in intensive
care admission, but is beyond the scope of this paper.
Advanced modalities
1. Doppler and colour Doppler
techniques
Doppler US is used to assess the signal from visceral
vessels that supply the gastrointestinal (GI) tract and smaller vessels within
the intestinal wall. Although the technique cannot be used to assess capillary
flow, it can be used to analyse all the major visceral vessels e.g. renal,
hepatic, mesenteric. It has to be noted that normal bowel wall perfusion cannot
be demonstrated by colour or power Doppler. The presence of flow in the bowel
wall points towards pathologic perfusion (e.g. hyperaemia in actively inflamed
segments, as seen in appendicitis).
Commonly used measurements which can be performed
include:
- Systolic, diastolic and mean velocities
- Pulsatility index
- Resistance index (peak systolic velocity –
end diastolic velocity)/peak systolic velocity
- Blood flow volume
GI tract blood flow
Colour Doppler allows for the assessment of mural
flow, the absence of which is a sign of ischaemia. Unfortunately, this finding
is only reported in 20–50% of patients with a proven diagnosis of ischaemic
colitis (Danse et al. 2000a; Danse et al. 2000b). Doppler US can show stenosis,
emboli, and thrombosis in the near visible parts of the coeliac trunk, the
superior mesenteric artery (SMA) and the inferior mesenteric artery (IMA). In
the early phase of bowel ischaemia, US examinations may show SMA occlusion,
hyperaemic segments and bowel spasm. Collateral vessels cannot be reliably
displayed using ultrasound. Systolic velocities of more than 250–300 cm/s are
sensitive indicators of severe mesenteric arterial stenosis (Hamada et al.
2014; Koenig et al. 2011). The spectral analysis of Doppler signals of arteries
supplying the GI tract (truncus coeliacus, superior and inferior mesenteric
arteries) and the vessels draining the intestine, can be used to estimate bowel
perfusion (see below). Assessment in transverse and longitudinal plane should
be performed. Low flow states can also be identified by the presence of spontaneous
contrast and turbulent flow in the large vessels.
Hepatic blood flow
Portal vein: the normal main portal vein (MPV) is gently
undulating with peak systolic velocities ranging between 20 cm/s and 40 cm/s. A
low flow velocity of <16 cm/s in addition to a calibre increase in the MPV
are diagnostic features of portal hypertension (PH). Further worsening of PH
leads to a to-and-fro flow pattern, whereby the nearly stagnant blood column in
the portal veins is seen to shift into and out of the liver with the
respiratory cycle. In the end stages, stagnation of the blood column can lead
to thrombosis or progress to a frank flow reversal or non-forward portal flow
(NFPF). This is considered to have grave prognostic significance, indicating severe
and irreversible liver failure (Wachsberg et al. 2002).
Hepatic vein: the normal flow is triphasic with two
hepatofugal phases related to atrial and ventricular diastole. Fibrotic or
inflammatory changes may create a monophasic flow pattern. Early waveform
changes in cirrhosis patients include spectral broadening and dampening of the
normal, retrograde, pre-systolic wave of the hepatic vein waveform. Later, the
normal triphasic waveform pattern may be diminished or replaced with a
monophasic pattern. Therefore the monophasic hepatic vein waveform indicates
relatively high portal pressures (Ralls 1990).
Hepatic artery: hepatic arterial resistance changes
with increasing portal pressure values, but hepatic arterial resistive indices
correlate poorly with the severity of cirrhosis and will not be further
discussed here.
Renal blood flow
Doppler US can be used to assess renal perfusion.
Normal resistive index (RI) is approximately 0.58 ± 0.10 and values >0.70
are considered to be abnormal. A renal Doppler RI may also help in detecting
early renal dysfunction or predicting short-term reversibility of acute kidney
injury (AKI) in critically ill patients. A recent meta-analysis suggested that
RI may be a predictor of persistent AKI in critically ill patients with a
pooled sensitivity and specificity of 0.83 (95% CI, 0.77-0.88) and 0.84 (95%
CI, 0.79-0.88) (Ninet et al. 2015).
Increased renal resistive index (RRI) has been proven
to be an independent predictor of worse cardiovascular and renal outcomes, especially
when combined with reduced glomerular filtration rate (GFR), thus providing a
useful diagnostic complement to the assessment of renal function in these
patients. High RRI has also been correlated with the presence of hypertensive
and atherosclerotic organ damage. Values >0.80 have been reported to be
predictive of all-cause mortality in chronic kidney disease patients and may
indicate impending renal transplant failure in this patient subset (Barozzi et
al. 2007; Guinot et al. 2013; Ninet et al. 2015; Schnell et al. 2012).
Gastrointestinal and urinary
tract sonography (GUTS) protocol
Gastrointestinal function can be assessed with US,
using a combination of anatomical, functional and blood flow evaluation
(Schmidt et al. 2005).
- Function:
peristalsis, bowel motility, gastroparesis, small bowel ileus, large bowel
paralysis
- Dimensions:
bowel dilatation, bowel obstruction, Ogilvie syndrome, bacterial
overgrowth, toxic megacolon, bowel wall oedema, abdominal wall oedema
- Collections:
bowel content (blood, liquid, air, solid), haematoma, gastrointestinal
bleeding, ascites
- Perfusion:
bowel ischaemia, hepatosplanchnic perfusion, shock state (spontaneous
contrast), renal resistive index, abdominal perfusion pressure (APP) =
mean arterial pressure (MAP)-intraabdominal pressure (IAP)
This approach is summarised by the GUTS
(Gastrointestinal and Urinary Tract Sonography) protocol (Figure 3). The
structured and stepwise approach may lead to improved practical management of
adult ICU patients with acute gastrointestinal injury (AGI), as graded by the
European Consensus Definitions (Reintam Blaser et al. 2012). Such a management
strategy has not been shown to improve patient outcome, however.
The European Consensus Definition of AGI suggests a
graded severity score:
- AGI
grade I represents a self-limiting condition with increased risk of
developing GI dysfunction or failure
- AGI
grade II (GI dysfunction) represents a condition requiring interventions
to restore GI function
- AGI
grade III (GI failure) represents a condition when GI function cannot be
restored with interventions
- AGI
grade IV represents a dramatically manifesting GI failure, which is
immediately life-threatening (e.g. abdominal compartment syndrome with
organ dysfunction) (Reintam Blaser et al. 2012).
Future works
Contrast-enhanced ultrasound
Contrast-enhanced ultrasound (CEUS) involves the use
of contrast agents containing gas-filled microbubbles administered
intravenously, producing an image with greater contrast and/or highlighting
more vascular areas. Although reasonably well established in radiology, and
more recently cardiology departments, its use in intensive care is in its
infancy. Unlike CT contrast agents, CEUS appears safe for patients with renal
dysfunction and the modality itself remains free of radiation exposure to
patients. Possible use within critical care includes enhanced echocardiography
and in blunt abdominal trauma to assess solid-organ injuries (Dietrich 2017).
Doppler analysis as a marker of fluid status and venous congestion
As mentioned, Doppler analysis of the vasculature of
specific abdominal organs allows for assessment of its perfusion. Some early
work showed that this modality could also be used as a marker of systemic
vascular congestion (Lewis et al. 1989).
Conclusion
This paper summarises the multiple uses of abdominal
US on the ICU and highlights future work and development. It must be remembered
however that despite the myriad of potential diagnosis, utilisation and
interpretation of such techniques requires training and experience.
Acknowledgements and conflicts
of interest
Jonny Wilkinson is a member of the International Fluid
Academy (IFA) faculty. Manu Malbrain is founding President of WSACS (The
Abdominal Compartment Society) and current Treasurer, he is also member of the
medical advisory Board of Getinge (former Pulsion Medical Systems) and Serenno
medical, and consults for ConvaTec, Acelity, Spiegelberg and Holtech Medical.
He is co-founder and member of the executive committee of the International
Fluid Academy (IFA).
Adrian
Wong is a member of the executive committee of the IFA.
Abbreviations
FAST
Focused Assessment with Sonography in Trauma
GI
gastrointestinal
POCUS
point-of-care ultrasound
US
ultrasound