Abstract
Objectives—To evaluate
the performance of liver and spleen stiffness measured by acoustic radiation
force impulse (ARFI) elastography for noninvasive assessment of liver fibrosis
and esophageal varices in patients with chronic hepatitis B virus.
Methods—Two hundred
sixty-four participants, of whom 60 were healthy volunteers (classified as
stage 0), 66 were patients with chronic hepatitis B who had undergone liver
biopsy, and 138 were patients with hepatitis B-related cirrhosis, were enrolled
in this study. Median liver and spleen stiffness values (meters per second) from
10 successful measurements per participant were obtained. Patients with
cirrhosis were examined by upper endoscopy.
Results—Significant linear correlations were found between liver (Spearman ρ= 0.87; P < .001) and spleen (Spearman ρ = 0.76; P < .001) stiffness and the fibrosis stage. Liver and spleen stiffness values increased as fibrosis progressed; however, overlaps in liver stiffness were detected in stages 0 and 1 and 1 and 2, and overlaps in spleen stiffness were observed in stages 0 and 1, 1 and 2, and 2 and 3. Liver stiffness cutoff values were 1.69 m/s for predicting stage 3 or greater (area under the receiver operating characteristic curve [AUROC] = 0.99) and 1.88 m/s for stage 4 (AUROC = 0.97). The spleen stiffness cutoff value was 2.72 m/s for stage 4 (AUROC = 0.96). Liver stiffness was not correlated with the varix grade, whereas a significant linear correlation (Spearman ρ = 0.65; P < .001) between spleen stiffness and the varix grade was found. The optimal spleen stiffness cutoff value for predicting varices was 3.16 m/s (AUROC = 0.83).
Conclusions—Liver and spleen stiffness values measured by ARFI elastography are reliable predictors of liver fibrosis. Spleen stiffness measured by ARFI can be used as a non-invasive method for determining the presence and severity of esophageal varices; however, evidence to support a similar role for liver stiffness is lacking.
Hepatitis B,
which is caused by the hepatitis B virus (HBV), is a potential life-threatening
liver infection and the most serious type of viral hepatitis. As the World
Health Organization reported,1
approximately 2 billion people have been infected with HBV worldwide, and more
than 350 million people have chronic hepatitis B. In China
The
management and prognosis of chronic hepatitis B depend on the fibrosis stage.
To date, liver biopsy, an invasive technique, is still considered the reference
standard. However, its clinical application is limited because of its invasive
nature, potential severe complications, sampling errors, and interobserver and
intraobserver diagnostic discrepancies.4–6
In cirrhotic
patients, screening for esophageal varices is highly recommended and extremely
important because it is closely linked to the scheme of nonselective
beta-blocker therapy or endoscopic prophylaxis to prevent variceal bleeding.7 The
present screening method is endoscopy, which is performed every 2 to 3 years in
patients without esophageal varices, every 1 to 2 years in those with mild
varices, and annually in those with decompensated cirrhosis. However, this
method is invasive, expensive, and not easily accepted by patients.
For the
above-mentioned reasons, some noninvasive methods have been proposed to serve
as markers for evaluating of the degree of liver fibrosis and that of
esophageal varices, including serum markers,8–10
transient elastography,9–12
magnetic resonance elastography,13–15 and
acoustic radiation force impulse (ARFI) elastography.10,16,17
Acoustic
radiation force impulse imaging10,16,18 is a
novel technology based on conventional B-mode sonography. An acoustic push
pulse excites the tissue and produces shear waves that spread away from the
tissue. The propagation of the shear waves can be measured, and their speed
depends on the elasticity of the tissue. Therefore, ARFI provides numeric
measurements of tissue stiffness as the shear wave velocity, expressed as
meters per second. Publications have shown that liver stiffness values
correlated well with liver fibrosis staging determined by liver biopsy.18,19
Spleen stiffness measured by ARFI elastography in patients with chronic liver
disease has also been previously reported.16,17
However, those studies focused mostly on patients infected with the hepatitis C
virus (HCV).
The aims of
this study were to evaluate the accuracy of liver and spleen stiffness measured
by ARFI elastography for noninvasive assessment of liver fibrosis in patients
with chronic hepatitis B and to investigate whether liver and spleen stiffness
values are suitable predictors of the presence and severity of esophageal
varices.
Liver
and Spleen Stiffness Measurements
Liver and
spleen stiffness measurements were performed with an Acuson S2000 ultrasound
system equipped with virtual touch tissue quantification software (Siemens
Medical Solutions, Mountain View ,
CA
The interval
between these measurements and the liver biopsy that 66 of the participants
underwent ranged from 1 to 3 days (mean, 2.00 ± 0.84 days). Acoustic radiation
force impulse imaging was not immediately performed after biopsy because the
patients would not have been able to tolerate the pain caused by the biopsy and
scanning immediately after the procedure and because they needed to rest in the
dorsal decubitus position with a monitor for the first 6 hours.
The right
lobe was chosen because ARFI measurements of the right lobe are reportedly
potentially superior to those of the left lobe for diagnosis of liver fibrosis.20 A
point 2 to 3 cm under the liver capsule was chosen because a study of 3 points
under the liver capsule (0–1, 1–2, and 2–3 cm) found that the most reliable
liver elasticity values are obtained when ARFI measurements are made 2 to 3 cm
under the liver capsule.19
Discussion
The stage of
liver fibrosis can be quantified by ARFI elastography. Recent studies have
reported that measurement of liver stiffness using ARFI elastography is a
novel, accurate, and reliable noninvasive method for assessment of liver
fibrosis in patients with chronic liver disease.21,22 As
liver fibrosis progresses, hemodynamic and pathologic changes can be found in
the spleen, especially in late stages of fibrosis. One study23 found
that the size of the spleen increased as liver fibrosis progressed and was more
apparent in late stages. The density of the spleen changes in patients with an
enlarged spleen because of tissue hyperplasia, fibrosis, and portal and splenic
congestion.12
Such changes in the spleen are mechanical properties that can be quantified by
ARFI elastography. Therefore, we tried to show that liver and spleen stiffness
values are useful predictors for evaluation of liver fibrosis in chronic liver
disease and to evaluate their performance in predicting the presence of
esophageal varices in cirrhotic patients. Moreover, to our knowledge, a study
assessing liver fibrosis and esophageal varices specifically in HBV-infected
patients has not been reported previously.
Our data clearly
showed that liver stiffness correlated well with fibrosis. The stiffness
increased with the fibrosis stage. However, overlaps were detected between
stages 0 and 1 and stages 1 and 2. Similarly, a study of 112 patients with
chronic hepatitis C found overlaps between the consecutive stages F1 and F2 and
stages F2 and F3 (METAVIR scores).18
Another study of 103 patients with chronic hepatitis of different etiologies
observed overlaps between stages F0 and F1 and stages F3 and F4 (METAVIR
scores).24
These findings suggest that overlaps between consecutive stages occur
regardless of etiology. For the healthy volunteers (stage 0), the mean liver
stiffness value in our study was 1.13 ± 0.12 m/s which is comparable to those
published so far: 1.13 ± 0.23 m/s in healthy volunteers and 1.16 ± 0.17 m/s in
stage F0 patients (METAVIR score)10 and
1.15 ± 0.21 m/s in another group of healthy volunteers.25 For
cirrhotic patients, the mean liver stiffness value was 2.50 ± 0.50 m/s,
comparable to 2.38 ± 0.74 m/s in 81 patients with HCV or HBV infection10 and
2.552 ± 0.7782 m/s in patients with HCV infection.18
We discovered
high predictive values of liver stiffness for stage 3 or greater and stage 4,
which were similar to the findings in a study of patients with chronic
hepatitis C18
and another study of patients with different etiologies.21 The
cutoff value for stage 3 or greater was 1.69 m/s, which was the same as the
value in a study by Toshima et al22
including 79 patients with different etiologies and similar to the value in a
study by Lupsor et al18
including 112 patients with chronic hepatitis C (1.61 m/s). The cutoff value
for stage 4 was 1.88 m/s, which was slightly higher than the value in the study
by Toshima et al22 (1.79
m/s) and lower than the value in the study by Lupsor et al18 (2.0
m/s).
This study
compared the mean spleen stiffness values measured by ARFI elastography among
fibrosis stages 0 to 4. There was a correlation between spleen stiffness and
fibrosis (Spearman ρ = 0.76; P < .001). The spleen stiffness values
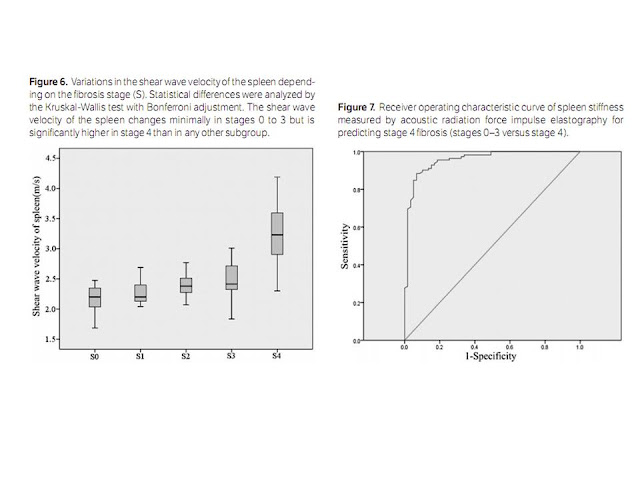
for stages 0 to 3 did not statistically differ, whereas stage 4 had a
significantly higher mean value than any other stage. In our study, the mean
spleen stiffness value in the healthy volunteers (stage 0) was 2.17 ± 0.24 m/s,
which was slightly higher than the previously reported value of 2.04 ± 0.28 m/s
in 15 healthy volunteers17 and lower
than the previously reported value of 2.44 m/s in 35 healthy volunteers.26 The
mean spleen stiffness value in cirrhotic patients (stage 4) was 3.24 ± 0.44
m/s, which was slightly higher than the value of 3.10 ± 0.55 m/s in a study by
Bota et al.17
To date, only
2 reports have analyzed spleen stiffness for predicting cirrhosis. One17
showed a cutoff value of 2.55 m/s for predicting cirrhosis with a good AUROC
(0.91), and another16 showed
a cutoff value of 2.73 m/s (AUROC = 0.82). Our results showed a high predictive
value for the presence of cirrhosis (AUROC = 0.96), with the cutoff (2.72 m/s)
being higher than that in the first study and similar to that in the second.
In this
study, a combined analysis of liver and spleen stiffness measured by ARFI for
predicting the presence of cirrhosis revealed that the sensitivity and
specificity were higher when one of the methods in the combined analysis
yielded positive results than when only one method was used, and the
specificity was significantly elevated when both methods yielded positive
results.
In cirrhotic
patients, a severe consequence is portal hypertension, which is a contributing
factor to the formation of esophageal varices and a direct cause of variceal
hemorrhage. Publications have reported that liver stiffness measured using
1-dimensional transient elastography (Fibroscan) showed a statistically
significant association with the hepatic venous pressure gradient.27–29 Some
studies found that liver stiffness measured by 1-dimensional transient
elastography was correlated with the esophageal varix grade,30
whereas others showed that liver stiffness correlated with the presence of
varices but showed a weak correlation with the varix size31 or
none at all.27
Acoustic radiation force impulse elastography is a 2-dimensional elastographic
technique. To our knowledge, there have been no reports about the correlation
between liver stiffness measured by ARFI and the hepatic venous pressure
gradient or between liver stiffness measured by AFRI and the presence of
esophageal varices. Only 1 study17
reported a correlation between spleen stiffness measured by ARFI and esophageal
varices, in which the authors observed no significant differences in the mean
spleen stiffness values between patients with and without varices of between
those with different varix grades.
Our data on
this issue were confusing. We found a correlation between spleen stiffness and
the varix stage, and there was a significant difference between patients with
and without varices. We determined a cutoff value of 3.16 m/s for predicting
the presence of varices (AUROC = 0.83). We also found a significant difference
between grades 2 and 3, but unfortunately, we were not able to distinguish the
mean spleen stiffness values between grades 1 and 2. These results may be
explained by 3 possible reasons. First, endoscopy was not performed by the same
physicians, and the assessment of the varix grade was subjective. Second, not
all patients consented to undergo endoscopy and ARFI elastography on the same
day; the interval between spleen stiffness measurements and endoscopy ranged
from 0 to 30 days, whereas the longest interval in the report by Bota et al17
reached up to 6 months. Third, the distribution of patients according to varix
grades was unequal.
Our article
clearly suggests that there was no correlation between liver stiffness measured
by ARFI and the esophageal varix grade, and no significant difference was found
among the varix grades. The different results between liver and spleen
stiffness for assessment of varices may be explained by 2 possible reasons. First,
we discovered that the cirrhotic patients with elevated ALT and AST levels had
higher liver stiffness values than those with normal ALT and AST levels,
whereas the difference was not significant for the spleen stiffness values
between the patients with normal ALT and AST levels and those with elevated ALT
and AST levels. Some publications have reported a correlation between liver
stiffness values measured by ARFI and necroinflammation.18,21,32 These
reports indicate that elevated ALT and AST levels may affect liver but not
spleen stiffness values, which may be the major factor accounting for the
above-mentioned findings. Second, the unequal distribution of patients
according to varix grades may have led to different numbers in each subgroup.
___________________________
ARFI
elastography có thể định lượng được các giai đoạn của xơ hoá gan. Các nghiên
cứu gần đây đã thông báo rằng đo độ cứng gan bằng ARFI elastography là một
phương pháp mới không xâm lấn, chính xác và đáng tin cậy của xơ hoá gan ở những
bệnh nhân gan mạn tính. Khi xơ hoá gan tiến triển, thay đổi huyết động học và
bệnh lý có thể thấy được ở lách, đặc
biệt là trong giai đoạn cuối của xơ hoá. Một nghiên cứu cho thấy kích thước
của lách tăng khi xơ hoá gan tiến triển
và rõ hơn trong giai đoạn cuối. Mật độ của thay đổi lách ở những bệnh nhân lách
to vì tăng sản mô, xơ hóa, và sung huyết lách và tĩnh mạch cửa. Những thay đổi lách như vậy là những đặc tính cơ học định lượng được bởi ARFI elastography. Vì vậy,
chúng tôi đã cố gắng chứng tỏ các giá trị độ cứng gan và lách là các yếu tố tiên
đoán hữu ích cho việc đánh giá xơ hóa gan trong bệnh gan mạn tính và đánh giá
hiệu suất của chúng trong tiên đoán của dãn tĩnh mạch thực quản ở bệnh nhân chai
gan. Hơn nữa, theo chúng tôi được biết, nghiên cứu đánh giá xơ hoá gan và dãn
tĩnh mạch thực quản đặc biệt ở bệnh nhân nhiễm HBV chưa từng được báo cáo.
Dữ liệu của
chúng tôi rõ ràng cho thấy độ cứng gan tương quan với xơ hóa. Độ cứng tăng lên theo
giai đoạn xơ hóa. Tuy nhiên, có chồng lấp giữa giai đoạn 0 và 1 và giai đoạn 1
và 2. Tương tự như vậy, một nghiên cứu ở 112 bệnh nhân viêm gan C được tìm thấy
có trùng lặp giữa các giai đoạn liên tiếp F1 và F2 và giai đoạn F2 và F3 (tính
điểm METAVIR). Một nghiên cứu khác của 103 bệnh nhân viêm gan mạn với nguyên
nhân khác nhau có chồng lấp giữa giai đoạn F0 và F1 và giai đoạn F3 và F4 (tính
điểm METAVIR). Những phát hiện này gợi ý có xảy ra chồng chéo giữa các giai đoạn
liên tiếp bất kể do nguyên nhân nào. Đối với các tình nguyện viên khỏe mạnh
(giai đoạn 0), giá trị độ cứng gan trung bình trong nghiên cứu của chúng tôi là
1,13 ± 0,12 m/s so sánh với những công bố cho đến nay:1,13 ± 0,23 m/s ở người
tình nguyện khỏe mạnh và 1,16 ± 0,17 m/s ở bệnh nhân giai đoạn F0 (tính điểm METAVIR) và
1,15 ± 0,21 m/s trong một nhóm khỏe mạnh tình nguyện. Đối với bệnh nhân chai gan,
giá trị trung bình độ cứng gan là 2,50 ± 0,50 m/s, tương đương với 2,38 ± 0,74
m/s ở 81 bệnh nhân nhiễm HCV hoặc HBV và 2,552 ± 0,7782 m/s ở những bệnh nhân nhiễm
HCV.
Chúng tôi phát
hiện ra giá trị tiên đoán cao của độ cứng của gan ở giai đoạn 3 hoặc cao hơn và
giai đoạn 4, tương tự như phát hiện trong một nghiên cứu khác ở bệnh nhân viêm
gan C mạn, và một nghiên cứu khác nữa của bệnh nhân có các nguyên nhân khác. Giá
trị cutoff cho giai đoạn 3 hoặc cao hơn là 1,69 m/s, tương tự như giá trị của
Toshima và cs với 79 bệnh nhân với các
nguyên nhân khác nhau và tương tự như giá trị của Lupsor và cs gồm 112 bệnh
nhân viêm gan C (1,61 m/s ). Giá trị cutoff cho giai đoạn 4 là 1,88 m/s, cao
hơn một ít so với nghiên cứu của Toshima và cs (1,79 m/s) và thấp hơn trong
nghiên cứu của Lupsor và cs (2,0 m/s).
Nghiên cứu này
nhằm so sánh các giá trị độ cứng lách trung bình đo bằng ARFI elastography giữa các
giai đoạn xơ hóa 0-4. Có tương quan giữa độ cứng lách và xơ hóa (Spearman ρ =
0,76; P <.001). Giá trị độ cứng lách cho các giai đoạn 0-3 không có khác biệt
thống kê, trong khi giai đoạn 4 có giá trị trung bình cao hơn có ý nghĩa hơn
bất kỳ giai đoạn nào khác. Trong nghiên cứu của chúng tôi, giá trị độ cứng lách
trung bình của các tình nguyện viên khỏe mạnh (giai đoạn 0) là 2,17 ± 0,24 m/s,
đó là hơi cao hơn giá trị báo cáo trước đây của 15 người tình nguyện khỏe mạnh
là 2,04 ± 0,28 m/s và thấp hơn so với giá trị của 35 người tình nguyện khỏe
mạnh là 2,44 m/s đã báo cáo trước đó. Giá
trị độ cứng lách trung bình ở bệnh nhân chai gan (giai đoạn 4) là 3,24 ± 0,44
m/s, cao hơn so với giá trị 3,10 ± 0,55 m/s trong một nghiên cứu Bota và cs.
Cho đến nay, chỉ
có 2 báo cáo đã phân tích độ cứng lách để tiên đoán xơ gan. Một nghiên cứu cho
giá trị cutoff là 2,55 m/s để dự đoán xơ
gan với một AUROC tốt (0,91), và bài khác cho giá trị cutoff là 2,73 m/s (AUROC
= 0,82). Kết quả của chúng tôi cho giá trị tiên đoán cao cho sự hiện diện của chai
gan (AUROC = 0,96), với cutoff (2,72 m/s) cao hơn nghiên cứu đầu tiên và tương
tự như lần thứ hai.
Trong nghiên cứu
này, một phân tích kết hợp của độ cứng gan và lách đo bằng ARFI để tiên đoán chai
gan cho thấy rằng độ nhạy và độ đặc hiệu cao hơn khi phương pháp
phân tích kết hợp mang lại kết quả dương tính hơn là chỉ có một phương pháp được sử dụng, và độ
đặc hiệu tăng đáng kể khi cả hai phương pháp mang lại kết quả dương tính.
Ở bệnh nhân chai
gan, hậu quả nghiêm trọng là cao áp tĩnh mạch cửa, là một yếu tố góp phần vào
sự hình thành dãn tĩnh mạch thực quản và là nguyên nhân trực tiếp gây xuất
huyết dãn tĩnh mạch thực quản. Y văn cho thấy đo độ cứng gan bằng cách
sử dụng 1-D elastography thoáng qua (FibroScan) có kết hợp có ý nghĩa
thống kê với gradient áp lực tĩnh mạch gan [hepatic venous pressure gradient]. Một số nghiên cứu thấy rằng độ
cứng gan đo bằng FibroScan có tương quan với giai đoạn dãn tĩnh mạch thực quản,
trong khi những khảo sát khác cho thấy độ cứng gan liên quan với có dãn tĩnh
mạch thực quản, nhưng có tương quan yếu với kích thước dãn tĩnh mạch hoặc không
có tương quan nào hết. Đo đàn hồi ARFI
là kỹ thuật đo đàn hồi 2 chiều. Theo chúng tôi biết, chưa có báo cáo về tương
quan giữa độ cứng gan đo bởi ARFI và gradient áp lực tĩnh mạch gan hoặc giữa độ
cứng gan đo bằng AFRI với hiện diện của dãn tĩnh mạch thực quản. Chỉ có 1 báo
cáo về tương quan giữa độ cứng lách đo bằng ARFI và dãn tĩnh mạch thực quản,
trong đó các tác giả quan sát thấy không có sự khác biệt đáng kể trong giá trị
độ cứng lách trung bình giữa các bệnh nhân có và không có dãn tĩnh mạch và giữa
những người có giai đoạn dãn tĩnh mạch thực quản khác nhau.
Dữ liệu của
chúng tôi về vấn đề này là khó hiểu. Chúng tôi thấy có tương quan giữa độ cứng
lách và giai đoạn dãn tĩnh mạch thực quản, và có khác biệt có ý nghĩa giữa bệnh nhân có và
không có dãn tĩnh mạch. Chúng tôi xác định một giá trị cutoff là 3,16 m/s để dự
đoán sự hiện diện của dãn tĩnh mạch (AUROC = 0,83). Chúng tôi cũng tìm thấy có
khác biệt đáng kể giữa giai đoạn 2 và 3, nhưng thật không may, không thể phân
biệt các giá trị độ cứng lách trung bình giữa giai đoạn 1 và 2. Những kết quả
này có thể được giải thích bởi 3 khả năng. Một, nội soi không được thực hiện bởi
các bác sĩ, và việc đánh giá giai đoạn dãn tĩnh mạch thực quản là chủ quan. Thứ
hai, không phải tất cả các bệnh nhân đồng ý được nội soi và ARFI elastography
trong cùng một ngày, khoảng thời gian giữa đo độ cứng lách và nội soi khoảng
0-30 ngày, trong khi khoảng thời gian dài nhất trong báo cáo của Bota và cs là
6 tháng. Thứ ba, phân bố của bệnh nhân theo giai đoạn dãn tĩnh mạch thực quản không
đồng đều.
Bài báo này của
chúng tôi, cho thấy rõ không có mối tương quan giữa độ cứng gan đo bằng ARFI với
giai đoạn dãn tĩnh mạch thực quản, và không có khác biệt có ý nghĩa giữa các
giai đoạn dãn tĩnh mạch thực quản. Các kết quả khác nhau giữa độ cứng gan và
lách trong đánh giá dãn tĩnh mạch thực quản có thể được giải thích bằng 2 khả
năng. Một, chúng tôi phát hiện ra rằng các bệnh nhân chai gan với mức ALT và
AST cao có giá trị độ cứng gan cao hơn những người có mức ALT và AST bình
thường, trong khi khác biệt giá trị độ cứng lách không có ý nghĩa giữa các bệnh
nhân có mức độ ALT và AST bình thường và những bệnh nhân có ALT và AST cao. Một
số báo cáo cho thấy có tương quan giữa giá trị độ cứng gan ARFI và viêm hoại tử
[necroinflammation]. Các báo cáo này chỉ ra rằng mức ALT và AST cao có thể ảnh
hưởng đến gan, nhưng không ảnh hưởng đến giá trị độ cứng lách, có thể là nhân
tố chính cho phát hiện đề cập ở trên. Thứ hai, phân bố các bệnh nhân không đồng
đều theo giai đoạn dãn tĩnh mạch thực quản có thể dẫn đến khác biệt về số lương
trong mỗi phụ nhóm.
Để kết luận, giá
trị độ cứng gan và lách đo bằng ARFI elastography là yếu tố tiên đoán đáng tin
cậy của xơ hoá gan, đặc biệt là đối với những bệnh nhân có nguy cơ cao hoặc
không sẵn sàng để sinh thiết gan. Độ cứng lách có thể được dùng như một phương
tiện không xâm lấn để tiên đoán có và giai đoạn của dãn tĩnh mạch thực quản và
cũng có thể có giá trị cho các bệnh nhân từ chối làm nội soi. Hơn nữa, các
nghiên cứu sâu hơn về độ cứng gan và lách nhằm đánh giá xơ hoá gan và dãn tĩnh
mạch thực quản trong một dân số lớn hơn đã được lên kế hoạch.