Abstract
AIM: To assess whether acoustic radiation force impulse (ARFI)
elastography can differentiate normal from pathological thyroid parenchyma.
METHODS: We evaluated 136 subjects (mean age 45.8 ± 15.6 years, 106
women and 30 men): 44 (32.3%) without thyroid pathology, 48 (35.3%) with
Basedow-Graves’ disease (GD), 37 (27.2%) with chronic autoimmune thyroiditis
(CAT; diagnosed by specific tests), 4 (2.9%) with diffuse thyroid goiter and 3
(2.2%) cases with thyroid pathology induced by amiodarone. In all patients, 10
elastographic measurements were made in the right thyroid lobe and 10 in the
left thyroid lobe, using a 1-4.5 MHZ convex probe and a 4-9 MHz linear probe,
respectively. Median values were calculated for thyroid stiffness and expressed
in meters/second (m/s).
RESULTS: Thyroid stiffness (TS) assessed by means of ARFI in healthy
subjects (2 ± 0.40 m/s) was significantly lower than in GD (2.67 ± 0.53 m/s) (P
< 0.0001) and CAT patients (2.43 ± 0.58 m/s) (P = 0.0002), but the
differences were not significant between GD vs CAT patients (P =
0.053). The optimal cut-off value for the prediction of diffuse thyroid
pathology was 2.36 m/s. For this cut-off value, TS had 62.5% sensitivity, 79.5%
specificity, 87.6% predictive positive value, 55.5% negative predictive value
and 72.7% accuracy for the presence of diffuse thyroid gland pathology (AUROC =
0.804). There were no significant differences between the TS values obtained
with linear vs convex probes and when 5 vs 10 measurements were
taken in each lobe (median values).
CONCLUSION: ARFI seems to be a useful method for the assessment of
diffuse thyroid gland pathology.
Keywords: Acoustic
radiation force impulse elastography, Thyroid stiffness, Thyroid pathology
INTRODUCTION
Clinical evaluation through thyroid palpation is the classical method
for assessing this superficial gland. In the last years, elastography has been
developed as a new dynamic technique that uses ultrasound waves for the
evaluation of tissue stiffness. The principle of ultrasound elastography is
that compression of the examined tissue induces less strain in hard tissues
than in soft ones. The ultrasound probe manually or automatically produces an
acoustic “push” pulse that generates shear-waves which propagate into the
tissue. The propagation speed increases with fibrosis[1,2].
Recently, several studies have assessed the value of different types of
elastography (transient elastography, real time elastography or acoustic
radiation force impulse elastography) for the evaluation of liver stiffness in
an attempt to replace liver biopsy. Elastographic methods are also used for the
assessment of focal lesions or of diffuse pathologies (especially chronic
hepatopathies)[3-8]. Many studies have
proved these methods to be valuable, especially for the diagnosis of advanced
fibrosis in diffuse liver diseases[7,9-14].
Considering the analogy of the two parenchymatous organs, liver and
thyroid, we tried to assess whether ultrasound-based elastography by means of
the acoustic radiation force impulse (ARFI) technique could be useful for the
evaluation of thyroid diffuse pathology.
The aim of our paper was to see whether, by using ARFI elastography, we
can differentiate a normal thyroid from a pathological one (considering only
diffuse thyroid diseases) and secondly, to establish technical parameters for
thyroid stiffness (TS) evaluation using ARFI elastography.
MATERIALS AND METHODS
We evaluated 136 subjects (mean age 45.8 ± 15.6 years, 106 women and 30
men): 44 (32.3%) without thyroid pathology, 48 (35.3%) with Basedow-Graves’
disease (GD), 37 (27.2%) with chronic autoimmune thyroiditis (CAT), 4 (2.9%)
with diffuse thyroid goiter and 3 (2.2%) cases with thyroid pathology induced
by amiodarone. All patients agreed to participate in our study which was
approved by the local Ethics Committee.
The diagnosis of GD was based on the following criteria: thyrotoxicosis
at the beginning confirmed by low thyroid stimulating hormone (TSH), high FT4
and FT3; diffuse hypoechoic goiter on ultrasound; and high titers of
anti-TSH receptor antibodies. Some of the cases were evaluated by ARFI at the
onset of the disease and some while under antithyroid therapy.
The diagnosis of CAT was based on high titers of antithyroid antibodies
(anti-TPO and/or antiTg); diffuse hypoechogenity of the thyroid parenchyma on
ultrasound; and normal or low thyroid function. Some of the cases had goiters
(Hashimoto type) and some had a normal thyroid volume on ultrasound
examination. All amiodarone treated patients developed type II thyrotoxicosis,
diagnosed by means of established criteria[15].
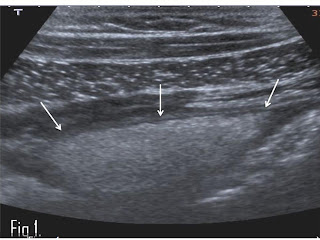
ARFI elastography was performed with a Siemens Acuson S2000™ ultrasound
system. In all patients, 10 elastographic measurements were taken in the right
thyroid lobe (RTL) and 10 in the left thyroid lobe (LTL) using a convex probe
of 1-4.5 MHz. Median values were calculated and expressed in meters/second
(m/s) (Figure (Figure11).
We calculated mean TS values in the RTL and LTL. To see whether the
probe type (linear or convex) influences TS measurements, in 45 patients we
performed 10 elastographic measurements each in the RTL and LTL, using a convex
probe of 1-4.5 MHz and a linear probe of 4-9 MHz, respectively. We also
calculated mean TS values for each probe (resulting from the median TS values
in RTL and LTL).
Data obtained from our cases were collected in a Microsoft Excel file,
the statistical analysis being performed using the MedCalc program. ARFI
measurements were numeric variables, so the mean values and standard deviation
were calculated. The t test was used to compare mean ARFI values of TS.
The diagnostic performance of ARFI elastography was assessed using ROC
curves that were constructed for prediction of thyroid pathology. Optimal
cut-off values were chosen to maximize the sum of sensitivity (Se) and specificity
(Sp). Se and Sp were calculated according to standard methods.
RESULTS
The mean TS values assessed by ARFI in normal and pathologic thyroid
for the LTL and RTL, and the median values for LTL plus RTL are presented in
Table 1
TS assessed by means of ARFI in healthy subjects was significantly
lower than in GD (P < 0.0001) and CAT patients (P = 0.0002),
but the differences were not statistically significant between GD vs CAT
cases (P = 0.053) .
The optimal cut-off value (in which the sum of Se and Sp was highest)
for the prediction of diffuse thyroid pathology was 2.36 m/s. For this cut-off
value, TS had 62.5% Se, 79.5% Sp, 87.6% positive predictive value (PPV), 55.5%
negative predictive value (NPV) and 72.7% accuracy for the presence of diffuse
thyroid pathology (AUROC = 0.804).
To obtain a Se > 90%, the best TS cut-off for predicting diffuse
thyroid pathology was 1.81 m/s (90.2% Se, 40.9% Sp, 76.1% PPV, 66.6% NPV and
74.2% accuracy).
To obtain a Sp > 90% the best TS cut-off assessed by ARFI
elastography was 2.53 m/s (54.3% Se, 90.9% Sp, 92.5% PPV, 48.7% NPV and 66.1%
accuracy).
If we compared mean TS values obtained by convex vs linear
probe, those obtained with the convex one were slightly higher, but not
significantly so, than those obtained with the linear one (2.17 ± 0.51 m/s vs
2.04 ± 0.43 m/s, P = 0.19) (Table 2). Also,
if only 5 ARFI measurements were performed in each thyroid lobe, their median
values were not significantly different from the median values of 10 ARFI
measurements (Table 2), in
normal as well as in diffuse thyroid disease.
Table 1:Mean thyroid stiffness values assessed by acoustic radiation force impulse in normal patients and in patients with diffuse thyroid pathology
Table 2:Mean acoustic radiation force
impulse thyroid stiffness values in normal and diffuse thyroid pathology, with
convex and linear probes, median of 10 measurements vs median of 5 measurements
Also, if only 5 ARFI measurements were performed, the TS assessed by
means of convex probe was slightly higher, but not significantly so, than those
obtained with the linear probe (2.11 ± 0.45 m/s vs 2.06 ± 0.38 m/s, P
= 0.63).
The mean ARFI values were significantly higher in patients with thyroid
pathology and low levels of TSH vs those with normal TSH (P =
0.03); however the mean ARFI values were similar in patients with low TSH vs
higher TSH P = 0.34) and in patients with normal TSH vs higher
TSH levels (P = 0.28) (Table 3). Also,
TS was not correlated with the TSH levels: Spearman r coefficient =
-0.157, P = 0.20.
Table 3:Thyroid stiffness acoustic
radiation force impulse measurements according to thyroid stimulating hormone
levels
DISCUSSION
GD is an autoimmune thyroid disorder characterized by diffuse goiter,
thyrotoxicosis, orbitopathy and occasionally, infiltrative dermopathy. The
clinical exam of the goiter by palpation reveals a parenchymatous elastic
consistency and a specific bruit. CAT is another autoimmune thyroid disease
that can induce goiter and/or thyroid dysfunction. Thyroid function is normal,
low or rarely high. The classic form of CAT (Hashimoto’s disease) presents a
diffuse goiter with hard consistency at palpation. If GD is characterized by
circulating anti-TSH immunoglobulins, CAT expresses serum antithyroid
autoantibodies which, in time, damage the thyroid’s morphofunctionality.
In previously published papers, thyroid elastography has been used to
evaluate thyroid nodule stiffness in order to differentiate malignant from
benign ones[16-22], usually using real
time elastography (Hi RT-E). There is only one published study (also by our
group) that evaluated thyroid stiffness by means of ARFI elastography in a
group of 74 subjects, as a predictor of diffuse thyroid pathology[23].
ARFI elastography involves targeting an anatomical region to be
investigated for elastic properties with the use of an ROI cursor, while
performing real-time B-mode imaging. Tissue in the ROI area is mechanically
excited using short-duration (262 μs) acoustic pulses with a fixed transmit
frequency of 2.67 MHz to generate localized tissue displacement. The
displacement results in shear wave propagation away from the region of
excitation and is tracked using ultrasound correlation-based methods[1,2]. The shear wave
propagation velocity is proportional to the square root of tissue elasticity so
that the propagation speed increases with fibrosis. Using image-based
localization and a proprietary implementation of ARFI technology, shear wave
speed may be quantified. Results are expressed in m/s. Measurement value and
depth are also reported.
Considering that there are no manufacturer recommendations for TS
evaluation, we performed 10 ARFI measurements in each thyroid lobe, after which
a median value was calculated, similar to the evaluation of liver stiffness by
means of transient elastography (TE) or ARFI. Thereafter, we retrospectively
analyzed the results, when only the first 5 ARFI measurements were taken into
consideration. TS values assessed by means of ARFI were not statistically
significant different if 10 vs 5 ARFI measurements were performed in
each thyroid lobe (Table 2), so
that we can conclude that for TS assessment 5 measurements are enough.
In the practical evaluation of liver stiffness through elastographic
methods (TE or ARFI), the high level of aminotransferases modifies the values
obtained for liver stiffness[24-26]. For this reason, we
wanted to see if a modified thyroid function plays a role in the TS evaluation.
We found that ARFI values were not correlated with TSH: Spearman r coefficient
= -0.157, P = 0.20. Considering all the patients with thyroid pathology,
the mean ARFI values were significantly higher in patients with abnormal TSH,
as compared with those with normal TSH (Table 3).
In a very recently published study by Friedrich-Rust et al[20], ARFI was used for
the evaluation of 55 patients with 60 thyroid nodules. TS measured by ARFI in
the healthy tissue surrounding the nodule was compared to the nodules’
stiffness. While no significant difference in median velocity was found between
healthy thyroid tissue and benign thyroid nodules, a significant difference was
found between malignant thyroid nodules on the one hand, and healthy thyroid
tissue (P = 0.018) or benign thyroid nodules (P = 0.014) on the
other hand.
Other elastographic methods have been used for TS assessment. In such a
study, Bahn et al[27]
used magnetic resonance elastography (MRE) to evaluate TS in cases without
thyroid pathology (12 subjects), in patients with Hashimoto thyroiditis (5
subjects), in patients with benign thyroid nodules (8 subjects) and with malignant
thyroid nodules (2 subjects). Statistically significant differences were found
between TS values in normal subjects (1.9 ± 0.6 kPa at 100 Hz and 1.3 ± 0.5 kPa
at 80 Hz) and those with Hashimoto thyroiditis (2.8 ± 0.6 kPa at 100 Hz and 1.8
± 0.6 kPa at 80 Hz) (P = 0.004 at 100 Hz). In the same MRE study,
elastographic parameters could not differentiate benign from malignant thyroid
nodules in this small cohort of patients.
In our study, TS assessed by means of ARFI in healthy subjects was
significantly lower than in GD (P < 0.0001) and CAT patients (P
= 0.0002), but the differences were not statistically significant between GD vs
CAT patients (P = 0.053), meaning that even if we cannot differentiate
by means of ARFI patients with GD from those with CAT, ARFI elastography could
be used in clinical practice for differentiating normal thyroid from diffuse
disease of the thyroid, maybe even as a first-line method, immediately after
performing routine ultrasound examination of the gland.
ARFI elastography of the thyroid is feasible with either linear or
convex probes and 5 measurements in every lobe are enough (median values) for
an accurate assessment. ARFI evaluation seems to be a useful method for
predicting the presence of autoimmune diffuse thyroid pathology, with high Sp
and PPV (> 90%) for cut-off values > 2.53 m/s; being able to make a first
differentiation between a normal thyroid and diffuse thyroid diseases
immediately after ultrasound evaluation, thus opening a new field in thyroid
elastography.
------------------------
REVISION of GREY SCALE THYROID ULTRASOUND
With grey scale US
Espinasse (1983) and Gutekunst et al. (1989) were the first
to report abnormal thyroid ultrasound patterns, characterized by a diffuse low
echogenicity, in patients with Hashimoto’s thyroiditis and Graves’ disease. Marcocci et al. found a diffuse low echogenicity in the thyroids of
44/238 patients with goiter and circulating thyroid autoantibodies.
The degree of hypoechogenicity was significantly correlated
with the levels of circulating thyroid autoantibodies. While thyroid function
was normal in all 194 patients with normal thyroid echogenicity, hypothyroidism
was found in 64% of those with thyroid hypoechogencity. Histology of excised
thyroid tissue from patients who underwent surgery for tracheal decompression
showed diffuse lymphocytic infiltration in patients with thyroid
hypoechogenicity, while in patients with normal thyroid echogenicity a histological pattern of colloid goiter with focal thyroiditis was
found. These data clearly showed that thyroid hypoechogenicity was due to
diffuse thyroiditis and was correlated with hypothyroidism. In the same paper
59/90 (65%) patients with Graves’ disease were found to have a diffusely low
thyroid echogenicity. While diffuse lymphocytic infiltration accounts for
thyroid hypoechogenicity in Hashimoto’s thyroiditis, in Graves’
disease the hypoechogenic pattern may be due to reduced colloid content with
Increased cellularity and reduction of the cell–colloid interface and/or to the increased blood flow.
Using ultrasonography, Vitti et al. reported that about
70% of patients with Graves’ disease exhibit a low thyroid echogenicity.
Whatever the reason for thyroid hypochogenicity in Graves ’
patients, this pattern is significantly associated with a higher frequency of thyrotropin
receptor antibody [TRAb] positivity and with the relapse of hyperthyroidism.
The study group included 105 patients who underwent a course of methimazole
treatment. Thyroid ultrasonography was performed at diagnosis, and TRAb levels
were measured at the end of treatment. During a 6–18 month follow-up period
after methimazole treatment, 87/105 (83%) patients had relapse of
hyperthyroidism and 18/105 (17%) were in remission.
Recurrence of hyperthyroidism occurred in 71/76 (93%)
patients with thyroid hypoechogenicity and in 16/29 (55%) of those with normal
thyroid echogenicity.
Positive TRAb values at the end of methimazole treatment
were found in 59/76 (78%) patients with thyroid hypoechogenicity and in 12/29
(41%) patients with normal thyroid echogenicity. Sixty-five of eighty-seven
(74%) patients with relapse of hyperthyroidism and 6/18 (33%) of those who
remained euthyroid were TRAb-positive at the end of methimazole treatment. The
finding of thyroid hypoechogenicity at diagnosis had higher specificity (0.81)
and sensitivity (0.72) compared with TRAb positivity at the end of methimazole
treatment (0.74 and 0.66, respectively) for the prediction of relapse of
hyperthyroidism. Therefore, the evaluation of thyroid echographic pattern
promised to be a useful prognostic tool in patients with Graves’ disease.
Shieman et al.,
studying 53 patients with Graves’ disease, confirm that thyroid echogenicity is
lower in these patients compared with that in 100 euthyroid volunteers. In agreement with the data of Vitti et
al., significantly lower echogenicity was found in patients with elevated
TRAb levels and in those with active ophthalmopathy, suggesting that in some
way this echographic pattern is associated with a more active disease. The
merit of the paper of Shieman is the effort the authors made to obtain an objective measurement of thyroid echogenicity. To this
purpose, the thyroid images obtained with a 7.5MHz real-time transducer were
recorded, keeping the operating conditions constant, and in selected regions
of the thyroid the grey scale density was evaluated and translated into a
numerical scale. The intraassay and interassay variations of grey determination
were lesser than 5 %.
In the last few years (circa 2000), increasing evidence has been obtained
indicating that thyroid US can be very useful in the diagnostic approach to
thyroid autoimmune diseases, including Graves’ disease. At present, thyroid US
Thus, ultrasound imaging is likely to increase in importance
for diagnosis and follow-up of Graves’ disease and become an essential
technique for all thyroidologists.